
Medical network - February 16 recently, abbvie announced the 2016 results, annual net income of $25.638 billion, compared with $2015 in 22.859 billion, an increase of 12.2%. Among them, the Ada wood single resistance (merlot) took in $16.078 billion, was awarded the 2016 global drug sales champion. This is repairing the merlot since 2012 after clopidogrel (wave d) after 5 consecutive years was named "who" reputation around the world.
Back in 2015, for the global "who" first two competitive products. Ada wood single resistance (Humira) generated sales of around usd $14.293 billion and was ranked no. 1; And cloth of hepatitis c drug compound SuoFei god wei/Mr Reddy wei (Harvoni) sales were $13.864 billion, ranking second. Two products sales is less than $500 million.
Ada wood 2016 single sales of $16.078 billion, resistance and cloth of compound SuoFei wei/Mr Reddy wei sales of about $9 billion. Due to the sharp diving Harvoni sales (in the first nine months of 2016 sales of $7.441 billion), Harvoni has not Humira opponent.
Best-selling drug TOP 10 marks
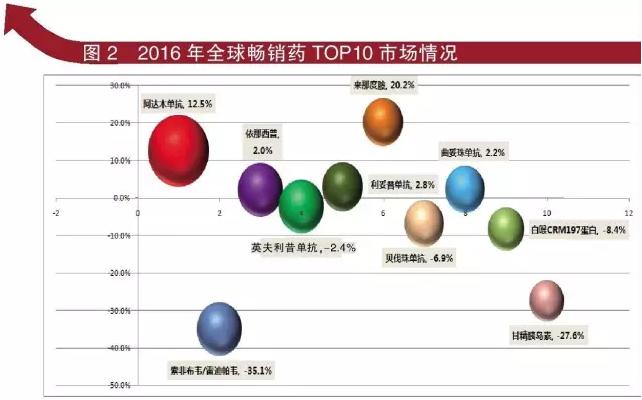
2016 global TOP 10 best-selling drug, respectively is: Ada wood sheet resistance, compound SuoFei cloth wei/Mr Wei reddy, infliximab (remicade, according to the heap, sheet resistance, rituxan, beacizumab bead sheet resistance, drug lenalidomide, by beads, diphtheria CRM197, insulin resistance.
In 2016 TOP ten best-selling drug sales have more than $5 billion, total sales of $81.409 billion, while the data for $2015 in 86.022 billion. In 2015, the world's best selling drugs, there are 2 kinds of sales of more than $10 billion, respectively is AbbVie (AbbVie) merlot (Humira) and Gilead (Gilead) Harvoni, sales is $14.293 billion and $13.864 billion respectively. In 2016, Gilead (Gilead) Harvoni shrink nearly $5 billion in sales.
2016 global TOP 10 best-selling drugs are: Abbott (Abbott laboratories), wood sheet resistance generated sales of around $16.078 billion, a 12.5% increase from the same period. Gilead (Gilead) compound SuoFei cloth wei/Mr Reddy wei sales were $9 billion, more fell 35.1%; Amgen (Amgen)/Pfizer (Pfizer) in accordance with the west's sales were $8.874 billion, a 2.0% increase from the same period. Pfizer (Pfizer) diphtheria CRM197 protein generated sales of around usd $5.718 billion and was fell 8.4%; Merck (Merck)/Johnson & Johnson (j&j) infliximab (remicade, / Mitsubis single sales were $8.7 billion, more fell 2.4%; Roche (Roche)/Chugai (foreign) company has three product, rituxan sales were $7.3 billion, a 2.8% increase from the same period, beacizumab bead single sales were $6.783 billion, fell 6.9%, by bead single sales were $6.782 billion, a 2.2% increase from the same period. Its drug lenalidomide (new) company sales were $6.974 billion, a 20.2% increase from the same period. Sanofi (Sanofi) of insulin sales were $5.2 billion, fell 27.2%.
The most beautiful and the most miserable
In 2016 the global TOP 10 most anticipated best-selling drug is Ada wood sheet resistance and drug lenalidomide two products, sales increase from the same period were over 10%, the drug lenalidomide rose more than 20%. "Who" Humira (Ada wood single) resistance performance is impressive, 2016 sales of $16.078 billion. Revlimid (drug lenalidomide) sales of nearly $7 billion.
The most miserable of the two products is a compound SuoFei cloth wei/redi pavese and insulin, are relatively fell more than 20%, especially of gilead Harvoni HCV drugs by the time fall by more than 35.0%.
"Who" short-term difficult to break the sales record
Ada developed by abbvie wood sheet resistance, in December 31, 2002, the FDA approval, on September 8, 2003 won European approval of EMA, on April 16, 2008 PMDA, Japan has approved, on February 26, 2010 listed on the CFDA granted, and by the abbvie sales in the United States, Europe, Japan and China market, commodity called "Humira.
Humira as abbvie's flagship product, is the world's first approved the anti-tumor necrosis factor of TNF alpha drugs, the drug is the world's best-selling anti-inflammatory drugs. Listed in 13 years, it has won approval of more than 90 countries around the world, 14 worldwide approved indications, market 10 indication approved in the United States, the world more than 980000 patients were treated with Humira.
Humira is abbvie super blockbuster products, for five consecutive years held the "who", the drug has sales of more than $14 billion in 2015, the clinical indications steadily widening, as the product again in 2016, sales break through the $16 billion mark. Recently, while facing patent cliff near and Ada wood single analogue test of the forthcoming, but these factors overall influence on Humira sales co., LTD.
Current global Ada wood single biological resistance similar drug development momentum of the related biological 2022 years ago the possibility of a similar drug on the market in Europe and the United States. Though Ada wood sheet resistance in recent years has maintained a strong momentum of sales growth, slow economic growth but probably around 2018. With analysts predict that Humira plans to hit the $18 billion mark in 2020. Whether these creatures like medicine can be listed as soon as possible, Ada wood abbvie single sales record in the short term is difficult to break.
The drug lenalidomide soared 20%
Drug lenalidomide its development, by the United States commodity called "Revlimid. The drug by the FDA in 2003 as a rare diseases drugs and into the fast channel of examination and approval, 2005 approved for the treatment of myelodysplastic syndrome (MDS), and then in 2008 approved for the treatment of multiple myeloma (MM). In addition, the drug lenalidomide also has therapeutic effect for a variety of leukemia and solid tumor, in June 2013, approved by FDA drug lenalidomide treatment cell lymphoma new indications. The current drug lenalidomide indications continues to expand, is expected to expand the market space.
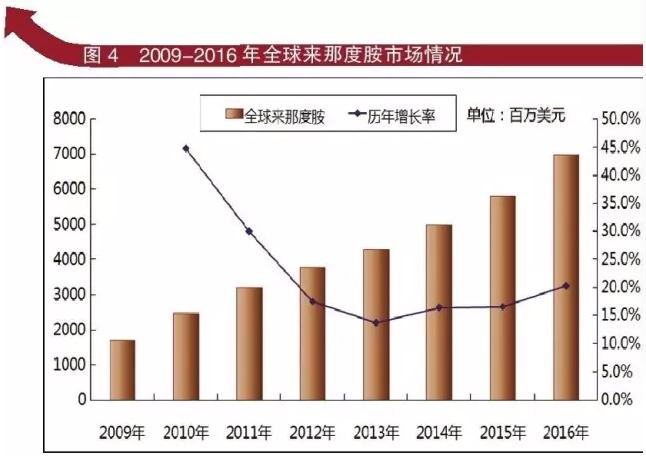
The drug lenalidomide is the new base company developed a new generation of blood disease treatment. As a rising star in the new company, relying on the drug lenalidomide occupy American medicine. According to the new base 2016 results analysis, drug lenalidomide relying on strong growth momentum, annual net sales revenue of $11.185 billion, compared with $2015 in the same period in 2015 increased by 20%, has successfully entered the biopharmaceutical company billion-dollar club. Drug lenalidomide sales revenue reached $2016 in 7 billion, the drug is approved in February 2015, the first line therapy for multiple myeloma, and then the listed drugs are more requirements and drug lenalidomide combination, which greatly increases the drug lenalidomide target patients. The drug lenalidomide has become one of the most popular blockbuster products around the world.
According to the world's bestselling drug data statistics, the drug lenalidomide has amounted to 2015 sales of $2015, 2016 annual sales of $6.974 billion, a 20.2% increase from the same period. From the point of sales data from 2006 to 2016, the product annual compound growth rate of 22.3%. The drug in 2015 global sales of $5 billion, into the world's ten biggest blockbuster drugs, no. 10. In 2016 nearly $7 billion in global sales, ranking sixth, for the 2016 top 10 fastest growing in the blockbuster products products. Its expectation to the drug lenalidomide is higher, its expected 2020 sales reached a peak of $15 billion, is likely to become the new "who" in the world.
Has several domestic enterprise research and development of generic drug lenalidomide, only Beijing double heron medicine into the production stage. Double heron pharmaceutical drug lenalidomide has site verification through the clinical trial data and for urgent clinical needs, first apply for reasons such as production include priority review, and in 2016, on September 27, complete the NDA review. In addition, the double heron medicine also for drug lenalidomide has carried on the patent challenges Chinese patents, relevant patent has been authorized. Which can be pushed, the double heron pharmaceutical industry should be the head of the drug lenalidomide imitation manufacturers. |
