Medical network - on February 15, New Year new atmosphere, in January, 2017 CDE drug registration acceptance, according to the total amount on the whole stable, did not appear as usual for the lunar New Year holiday causes significant monthly decline phenomenon. Which new drug related registration accepted amount compared with the previous month and growth, especially in the case of localization of medicine clinical application and listed on the application, not only in Numbers continue to rebound, also a major breakthrough on varieties, quality, a New Year is a good start.
Clinical application: the medicine continue to rebound
Data of domestic and imported from two aspects, 1 month for CDE to accept the new drug clinical trials to apply for a total of 30 (note: the same firms declare the same generic drugs for 1 meter, the same below), in which the drug related 21, 8 related biological products and traditional Chinese medicine and natural medicine related to 1. Contrast the half-year monthly data as you can see, the chemical drugs clinical applications fell in September 16 years after the slow rebound, this month is 4 months remain stable or rising trend.
Data sources: China's new drug research and development monitoring database of CPM
CDE this month to accept the new drug application in the clinical trials, including 11 class 1 new drug, eight of them for the first time in our country, put forward the clinical application of drugs, at the same time there are 4 companies new to class 1 new drug research and development, declaration of completed his first new class of drugs (table 1).
Table 1. CDE accepts the clinical application of 1 January 2017 kinds of chemical drugs
New drug applications: class 1 new drug darrow rui wei
In January for CDE to accept the new drug applications a total of 9 (table 2), for nearly a year to accept the amount of monthly average of nearly 2 times. Among them, the domestic new drugs listed apply for 4 pieces, including class 1 new drug hepatitis c treatment darrow rui wei, 2.2 amend new drug gliclazide sustained-release capsules and sodium phosphate salt and biological products polyethylene glycol recombinant variant integration interferon injection. Including darrow rui wei direct antiviral drugs for the treatment of hepatitis c (DAA), from research and development drug firms song li pharmaceutical industry in the form of patent licensing imported from multinational pharmaceutical companies roche pharmaceuticals. Such as eventually to go public, for domestic HCV drugs market and domestic new drug research and development mode innovation is of great significance.
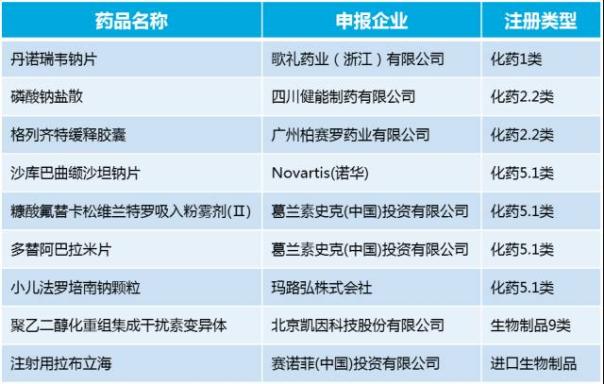
Table 2. CDE in January of 2017 to accept the new drug applications
For generic drugs registration: new 3 classes to record highs
In January for CDE of generics to apply for a total of 27, from basic flat. Among them according to the new registered drug classification filed for three new classes for generic drugs registration has 8 pieces, a record high since August for the first time in 16 years (table 3).
Table 3. CDE in January of 2017 to accept the new three kinds of generic drugs
|
